This method uses Counts done before and after deworming to allow you to monitor for drug-resistance.
The graduated cylinder was originally made for horses, because the worm burden in goats is so much higher we need to modify the technique for goats.
1) Collect your fecal sample and run the sample soon after collection. If you wait too long the eggs will hatch and you will not be able to count them.
2) Fill the cylinder with fecal flotation solution to half way between to two lines. (This will be 28mls.) (A simple flotation solution can be made by dissolving a pound of sugar in 1 ½ cups of water.)
3) Crush your nanny berries up and put enough of the crushed sample into the cylinder to raise the level of fluid to the top of the two lines (30ml total). Then stir well.
4) Take a sample of the mixture with a pipette and transfer it to one of the chambers of the McMaster slide. Repeat the procedure and fill the other chamber
5) Wait 30 sec then count the total number of eggs under both of the etched areas on the slide. Using a 10x lens and a 10x eyepiece on your microscope, focus first on the etched lines of the grid, then go down a tiny bit, the eggs will be floating just below the top of the chamber. Multiply the total number of eggs in the 2 chambers by 50, this is the eggs per gram (EPG).
6) Guidelines for Deworming
0-200 eggs–do not deworm
200-500 eggs–do not deworm but watch this goat, if she is sick for any reason, recount
500-1000 eggs–check mucous membrane color, if pale deworm, if pink and goat is feeling good do not deworm but watch closely
1000+ eggs–Always deworm
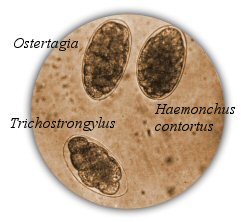

Fecal analysis kits can be purchased through vetsildes.com. Kits include the graduated cylinders, McMasters slides and syringes for pipetting. One kit contains enough to run 2 tests at a time and the cylinders and slides can be reused indefinitely.







Leave A Comment